The term “nonferrous” refers to all metals that have elements other than iron as its base or principal constituent. This group includes metals, such as aluminum, titanium, copper, and magnesium, as well as alloyed metals, such as Monel and Babbitt.
RELATED POSTS
Aluminum and Aluminum Alloys
Commercially pure aluminum is a white lustrous metal, which stands second in the scale of malleability, sixth in ductility, and ranks high in its resistance to corrosion. Aluminum combined with various percentages of other metals forms alloys, which are used in aircraft construction. Aluminum alloys with principal alloying ingredients are manganese, chromium, or magnesium and silicon show little attack in corrosive environments. Alloys with which substantial percentages of copper are more susceptible to corrosive action. The total percentage of alloying elements is seldom more than 6 or 7 percent in the wrought alloys.
Aluminum is one of the most widely used metals in modern aircraft construction. It is vital to the aviation industry because of its high strength-to-weight ratio and its comparative ease of fabrication. The outstanding characteristic of aluminum is its lightweight. Aluminum melts at the comparatively low temperature of 1,250 °F. It is nonmagnetic and is an excellent conductor.
Commercially pure aluminum has a tensile strength of about 13,000 psi, but rolling or other cold-working processes may approximately double its strength. By alloying with other metals, or by using heat-treating processes, the tensile strength may be raised to as high as 65,000 psi or to within the strength range of structural steel.
Aluminum alloys, although strong, are easily worked because they are malleable and ductile. They may be rolled into sheets as thin as 0.0017 inch or drawn into wire 0.004 inch in diameter. Most aluminum alloy sheet stock used in aircraft construction range from 0.016 to 0.096 inch in thickness; however, some of the larger aircraft use sheet stock that may be as thick as 0.356 inch.
The various types of aluminum may be divided into two general classes:
- Casting alloys (those suitable for casting in sand, permanent mold, or die castings)
- Wrought alloys (those which may be shaped by rolling, drawing, or forging).
Of these two, the wrought alloys are the most widely used in aircraft construction, being used for stringers, bulkheads, skin, rivets, and extruded sections.
Aluminum casting alloys are divided into two basic groups. In one, the physical properties of the alloys are determined by the alloying elements and cannot be changed after the metal is cast. In the other, the alloying elements make it possible to heat treat the casting to produce the desired physical properties.
A letter preceding the alloy number identifies the casting alloys. When a letter precedes a number, it indicates a slight variation in the composition of the original alloy. This variation in composition is simply to impart some desirable quality. For example, in casting alloy 214, the addition of zinc to improve its pouring qualities is indicated by the letter A in front of the number, thus creating the designation A214.
When castings have been heat treated, the heat treatment and the composition of the casting is indicated by the letter T, followed by an alloying number. An example of this is the sand casting alloy 355, which has several different compositions and tempers and is designated by 355-T6, 355-T51, or C355-T51.
Aluminum alloy castings are produced by one of three basic methods: sand mold, permanent mold, or die cast. In casting aluminum, it is important to note that in most cases different types of alloys must be used for different types of castings. Sand castings and die-castings require different types of alloys than those used in permanent molds.
Sand and permanent mold castings are parts produced by pouring molten metal into a previously prepared mold, allowing the metal to solidify or freeze and then removing the part. If the mold is made of sand, the part is a sand casting; if it is a metallic mold (usually cast iron), the part is a permanent mold casting. Sand and permanent castings are produced by pouring liquid metal into the mold, the metal flowing under the force of gravity alone.
The two principal types of sand casting alloys are 112 and 212. Little difference exists between the two metals in mechanical properties, since both are adaptable to a wide range of products.
The permanent mold process is a later development of the sand casting process, the major difference being in the material from which the molds are made. The advantage of this process is that there are fewer openings (called porosity) than in sand castings. The sand and the binder, which is mixed with the sand to hold it together, give off a certain amount of gas, that causes porosity in a sand casting.
Permanent mold castings are used to obtain higher mechanical properties, better surfaces, or more accurate dimensions. There are two specific types of permanent mold castings: permanent metal mold with metal cores, and semi-permanent types containing sand cores. Because finer grain structure is produced in alloys subjected to the rapid cooling of metal molds, they are far superior to the sand type castings. Alloys 122, A132, and 142 are commonly used in permanent mold castings, the principal uses of which are in internal combustion engines.
Die-castings used in aircraft are usually aluminum or magnesium alloy. If weight is of primary importance, magnesium alloy is used, because it is lighter than aluminum alloy. However, aluminum alloy is frequently used because it is stronger than most magnesium alloys.
Forcing molten metal under pressure into a metallic die and allowing it to solidify produces a die-casting; then the die is opened and the part removed. The basic difference between permanent mold casting and die-casting is that in the permanent mold process, the metal flows into the die under gravity. In the die-casting operation, the metal is forced under great pressure.
Die-castings are used where relatively large production of a given part is involved. Remember, any shape that can be forged, can be cast.
Wrought aluminum and wrought aluminum alloys are divided into two general classes: non-heat-treatable alloys and heat-treatable alloys.
Non-heat-treatable alloys are those in which the mechanical properties are determined by the amount of cold work introduced after the final annealing operation. The mechanical properties obtained by cold working are destroyed by any subsequent heating and cannot be restored except by additional cold working, which is not always possible. The “full hard” temper is produced by the maximum amount of cold work that is commercially practicable. Metal in the “as fabricated” condition is produced from the ingot without any subsequent controlled amount of cold working or thermal treatment. There is, consequently, a variable amount of strain hardening depending upon the thickness of the section.
For heat-treatable aluminum alloys, the mechanical properties are obtained by heat treating to a suitable temperature, holding at that temperature long enough to allow the alloying constituent to enter into solid solution, and then quenching to hold the constituent in solution. The metal is left in a supersaturated, unstable state and is then age hardened either by natural aging at room temperature or by artificial aging at some elevated temperature.
Wrought Aluminum
Wrought aluminum and wrought aluminum alloys are designated by a four-digit index system. The system is broken into three distinct groups: 1xxx group, 2xxx through 8xxx group, and 9xxx group (which is currently unused).
The first digit of a designation identifies the alloy type. The second digit indicates specific alloy modifications. Should the second number be zero, it would indicate no special control over individual impurities. Digits 1 through 9, however, when assigned consecutively as needed for the second number in this group, indicate the number of controls over individual impurities in the metal.
The last two digits of the 1xxx group are used to indicate the hundredths of 1 percent above the original 99 percent designated by the first digit. Thus, if the last two digits were 30, the alloy would contain 99 percent plus 0.30 percent of pure aluminum, or a total of 99.30 percent pure aluminum. Examples of alloys in this group are:
- 1100—99.00 percent pure aluminum with one control over individual impurities.
- 1130—99.30 percent pure aluminum with one control over individual impurities.
- 1275—99.75 percent pure aluminum with two controls over individual impurities.
In the 2xxx through 8xxx groups, the first digit indicates the major alloying element used in the formation of the alloy as follows:
- 2xxx—copper
- 3xxx—manganese
- 4xxx—silicon
- 5xxx—magnesium
- 6xxx—magnesium and silicon
- 7xxx—zinc
- 8xxx—other elements
In the 2xxx through 8xxx alloy groups, the second digit in the alloy designation indicates alloy modifications. If the second digit is zero, it indicates the original alloy, while digits 1 through 9 indicate alloy modifications. The last two of the four digits in the designation identify the different alloys in the group. [Figure]
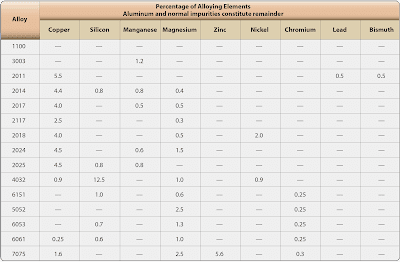 |
| Nominal composition of wrought aluminum alloys |
Effect of Alloying Element
1000 series: 99 percent aluminum or higher, excellent corrosion resistance, high thermal and electrical conductivity, low mechanical properties, excellent workability. Iron and silicon are major impurities.
2000 series: Copper is the principal alloying element. Solution heat treatment, optimum properties equal to mild steel, poor corrosion resistance unclad. It is usually clad with 6000 or high purity alloy. Its best-known alloy is 2024.
3000 series: Manganese is the principal alloying element of this group, which is generally non-heat treatable. The percentage of manganese that is alloy effective is 1.5 percent. The most popular is 3003, which is of moderate strength and has good working characteristics.
4000 series: Silicon is the principal alloying element of this group and lowers melting temperature. Its primary use is in welding and brazing. When used in welding heat-treatable alloys, this group responds to a limited amount of heat treatment.
5000 series: Magnesium is the principal alloying element. It has good welding and corrosion resistant characteristics. High temperatures (over 150 °F) or excessive cold working increases susceptibility to corrosion.
6000 series: Silicon and magnesium form magnesium silicide, which makes alloys heat treatable. It is of medium strength, good forming qualities, and has corrosion resistant characteristics.
7000 series: Zinc is the principal alloying element. The most popular alloy of the series is 6061. When coupled with magnesium, it results in heat-treatable alloys of very high strength. It usually has copper and chromium added. The principal alloy of this group is 7075.
Hardness Identification
Where used, the temper designation follows the alloy designation and is separated from it by a dash (i.e., 7075-T6, 2024-T4, and so forth). The temper designation consists of a letter indicating the basic temper, which may be more specifically defined by the addition of one or more digits. These designations are as follows:
- F—as fabricated
- O—annealed, recrystallized (wrought products only)
- H—strain hardened
- H1 (plus one or more digits)—strain hardened only
- H2 (plus one or more digits)—strain hardened and partially annealed
- H3 (plus one or more digits)—strain hardened and stabilized
The digit following the designations H1, H2, and H3 indicates the degree of strain hardening, number 8 representing the ultimate tensile strength equal to that achieved by a cold reduction of approximately 75 percent following a full anneal, 0 representing the annealed state.
Magnesium and Magnesium Alloys
Magnesium, the world’s lightest structural metal, is a silvery white material weighing only two-thirds as much as aluminum. Magnesium does not possess sufficient strength in its pure state for structural uses, but when alloyed with zinc, aluminum, and manganese, it produces an alloy having the highest strength-to-weight ratio of any of the commonly used metals.
Magnesium is probably more widely distributed in nature than any other metal. It can be obtained from such ores as dolomite and magnesite, as well as from seawater, underground brines, and waste solutions of potash. With about 10 million pounds of magnesium in one cubic mile of seawater, there is no danger of a dwindling supply.
Some of today’s aircraft require more than one-half ton of this metal for use in hundreds of vital spots. Some wing panels are fabricated entirely from magnesium alloys, weigh 18 percent less than standard aluminum panels, and have flown hundreds of satisfactory hours. Among the aircraft parts that have been made from magnesium with a substantial savings in weight are nose wheel doors, flap cover skin, aileron cover skin, oil tanks, floorings, fuselage parts, wingtips, engine nacelles, instrument panels, radio masts, hydraulic fluid tanks, oxygen bottle cases, ducts, and seats.
Magnesium alloys possess good casting characteristics. Their properties compare favorably with those of cast aluminum. In forging, hydraulic presses are ordinarily used, although, under certain conditions, forging can be accomplished in mechanical presses or with drop hammers.
Magnesium alloys are subject to such treatments as annealing, quenching, solution heat treatment, aging, and stabilizing. Sheet and plate magnesium are annealed at the rolling mill. The solution heat treatment is used to put as much of the alloying ingredients as possible into solid solution, which results in high tensile strength and maximum ductility. Aging is applied to castings following heat treatment where maximum hardness and yield strength are desired.
Magnesium embodies fire hazards of an unpredictable nature. When in large sections, its high thermal conductivity makes it difficult to ignite and prevents it from burning. It does not burn until the melting point of 1,204 °F is reached. However, magnesium dust and fine chips are ignited easily. Precautions must be taken to avoid this if possible. Should a fire occur, it could be extinguished with an extinguishing powder, such as soapstone or graphite. Water or any standard liquid or foam fire extinguisher causes magnesium to burn more rapidly and can cause explosions.
Magnesium alloys produced in the United States contain varying proportions of aluminum, manganese, and zinc. A letter of the alphabet designates these alloys, with the number 1 indicating high purity and maximum corrosion resistance.
Many of the magnesium alloys manufactured in the United States are produced by the Dow Chemical Company and have been given the trade name of Dow-metal™ alloys. To distinguish between these alloys, each is assigned a letter. Thus, we have Dow-metal™ J, Dow-metal™ M, and so forth.
Another manufacturer of magnesium alloys is the American Magnesium Corporation, a subsidiary of the Aluminum Company of America. This company uses an identification system like that used for aluminum alloys, with the exception that magnesium alloy numbers are preceded with the letters AM. Thus, AM240C is a cast alloy, and AM240C4 is the same alloy in the heat-treated state. AM3S0 is an annealed wrought alloy, and AM3SRT is the same alloy rolled after heat treatment.
Titanium and Titanium Alloys
An English priest named Gregot discovered titanium. A crude separation of titanium ore was accomplished in 1825. In 1906, enough pure titanium was isolated in metallic form to permit a study. Following this study, in 1932, an extraction process was developed and became the first commercial method for producing titanium. The United States Bureau of Mines began making titanium sponge in 1946, and 4 years later the melting process began.
The use of titanium is widespread. It is used in many commercial enterprises and is in constant demand for such items as pumps, screens, and other tools and fixtures where corrosion attack is prevalent. In aircraft construction and repair, titanium is used for fuselage skins, engine shrouds, firewalls, longerons, frames, fittings, air ducts, and fasteners.
Titanium is used for making compressor disks, spacer rings, compressor blades and vanes, through bolts, turbine housings and liners, and miscellaneous hardware for turbine engines. Titanium, in appearance, is like stainless steel. One quick method used to identify titanium is the spark test. Titanium gives off a brilliant white trace ending in a brilliant white burst. Also, moistening the titanium and using it to draw a line on a piece of glass can accomplish identification. This leaves a dark line similar in appearance to a pencil mark.
Titanium falls between aluminum and stainless steel in terms of elasticity, density, and elevated temperature strength. It has a melting point from 2,730 °F to 3,155 °F, low thermal conductivity, and a low coefficient of expansion. It is light, strong, and resistant to stress corrosion cracking. Titanium is approximately 60 percent heavier than aluminum and about 50 percent lighter than stainless steel.
Because of the high melting point of titanium, high temperature properties are disappointing. The ultimate yield strength of titanium drops rapidly above 800 °F. The absorption of oxygen and nitrogen from the air at temperatures above 1,000 °F makes the metal so brittle on long exposure that it soon becomes worthless. However, titanium does have some merit for short time exposure up to 3,000 °F where strength is not important. Aircraft firewalls demand this requirement.
Titanium is nonmagnetic and has an electrical resistance comparable to that of stainless steel. Some of the base alloys of titanium are quite hard. Heat treating and alloying do not develop the hardness of titanium to the high levels of some of the heat-treated alloys of steel. It was only recently that a heat-treatable titanium alloy was developed. Prior to the development of this alloy, heating and rolling was the only method of forming that could be accomplished. However, it is possible to form the new alloy in the soft condition and heat-treat it for hardness.
Iron, molybdenum, and chromium are used to stabilize titanium and produce alloys that quench-harden and age-harden. The addition of these metals also adds ductility. The fatigue resistance of titanium is greater than that of aluminum or steel.
Titanium becomes softer as the degree of purity is increased. It is not practical to distinguish between the various grades of commercially pure or unalloyed titanium by chemical analysis; therefore, the grades are determined by mechanical properties.
Titanium Designations
The A-B-C classification of titanium alloys was established to provide a convenient and simple means of describing all titanium alloys. Titanium and titanium alloys possess three basic types of crystals: A (alpha), B (beta), and C (combined alpha and beta). Their characteristics are:
- A (alpha)—all-around performance; good weld ability; tough and strong both cold and hot; and resistant to oxidation.
- B (beta)—bendability; excellent bend ductility; strong both cold and hot, but vulnerable to contamination.
- C (combined alpha and beta for compromise performances)—strong when cold and warm, but weak when hot; good bendability; moderate contamination resistance; excellent forge ability.
Titanium is manufactured for commercial use in two basic compositions: commercially-pure titanium and alloyed titanium. A-55 is an example of commercially-pure titanium. It has yield strength of 55,000 to 80,000 psi and is a general-purpose grade for moderate to severe forming. It is sometimes used for nonstructural aircraft parts and for all types of corrosion-resistant applications, such as tubing. Type A-70 titanium is closely related to type A-55 but has yield strength of 70,000 to 95,000 psi. It is used where higher strength is required, and it is specified for many moderately stressed aircraft parts. For many corrosion applications, it is used interchangeably with type A-55. Both type A-55 and type A-70 is weldable.
One of the widely-used titanium base alloys is designated as C-110M. It is used for primary structural members and aircraft skin, has 110,000 psi minimum yield strength, and contains 8 percent manganese.
Type A-110AT is a titanium alloy that contains 5 percent aluminum and 2.5 percent tin. It also has high minimum yield strength at elevated temperatures with the excellent welding characteristics inherent in alpha-type titanium alloys.
Corrosion Characteristics
The corrosion resistance of titanium deserves special mention. The resistance of the metal to corrosion is caused by the formation of a protective surface film of stable oxide or chemi-absorbed oxygen. Film is often produced by the presence of oxygen and oxidizing agents.
Corrosion of titanium is uniform. There is little evidence of pitting or other serious forms of localized attack. Normally, it is not subject to stress corrosion, corrosion fatigue, intergranular corrosion, or galvanic corrosion. Its corrosion resistance is equal or superior to 18-8 stainless steel.
Laboratory tests with acid and saline solutions show titanium polarizes readily. The net effect, in general, is to decrease current flow in galvanic and corrosion cells. Corrosion currents on the surface of titanium and metallic couples are naturally restricted. This partly accounts for good resistance to many chemicals; also, the material may be used with some dissimilar metals with no harmful galvanic effect on either.
Copper and Copper Alloys
Copper is one of the most widely distributed metals. It is the only reddish-colored metal and is second only to silver in electrical conductivity. Its use as a structural material is limited because of its great weight. However, some of its outstanding characteristics, such as its high electrical and heat conductivity, in many cases overbalance the weight factor.
Because it is very malleable and ductile, copper is ideal for making wire. It is corroded by salt water but is not affected by fresh water. The ultimate tensile strength of copper varies greatly. For cast copper, the tensile strength is about 25,000 psi, and when cold rolled or cold drawn, its tensile strength increases to a range of 40,000 to 67,000 psi.
In aircraft, copper is used primarily in the electrical system for bus bars, bonding, and as lock wire.
Beryllium copper is one of the most successful of all the copper base alloys. It is a recently developed alloy containing about 97 percent copper, 2 percent beryllium, and sufficient nickel to increase the percentage of elongation. The most valuable feature of this metal is that the physical properties can be greatly stepped up by heat treatment, the tensile strength rising from 70,000 psi in the annealed state to 200,000 psi in the heat-treated state. The resistance of beryllium copper to fatigue and wear makes it suitable for diaphragms, precision bearings and bushings, ball cages, and spring washers.
Brass is a copper alloy containing zinc and small amounts of aluminum, iron, lead, manganese, magnesium, nickel, phosphorous, and tin. Brass with a zinc content of 30 to 35 percent is very ductile, but that containing 45 percent has relatively high strength.
Muntz metal is a brass composed of 60 percent copper and 40 percent zinc. It has excellent corrosion-resistant qualities in salt water. Its strength can be increased by heat treatment. As cast, this metal has an ultimate tensile strength of 50,000 psi, and it can be elongated 18 percent. It is used in making bolts and nuts, as well as parts that come in contact with salt water. Red brass, sometimes termed “bronze” because of its tin content, is used in fuel and oil line fittings. This metal has good casting and finishing properties and machines freely.
Bronzes are copper alloys containing tin. The true bronzes have up to 25 percent tin, but those with less than 11 percent are most useful, especially for such items as tube fittings in aircraft.
Among the copper alloys are the copper aluminum alloys, of which the aluminum bronzes rank very high in aircraft usage. They would find greater usefulness in structures if it were not for their strength-to-weight ratio as compared with alloy steels. Wrought aluminum bronzes are almost as strong and ductile as medium carbon steel, and they possess a high degree of resistance to corrosion by air, salt water, and chemicals. They are readily forged, hot or cold rolled, and many react to heat treatment.
These copper base alloys contain up to 16 percent of aluminum (usually 5 to 11 percent), to which other metals, such as iron, nickel, or manganese, may be added. Aluminum bronzes have good tearing qualities, great strength, hardness, and resistance to both shock and fatigue. Because of these properties, they are used for diaphragms, gears, and pumps. Aluminum bronzes are available in rods, bars, plates, sheets, strips, and forgings.
Cast aluminum bronzes, using about 89 percent copper, 9 percent aluminum, and 2 percent of other elements, have high strength combined with ductility and are resistant to corrosion, shock, and fatigue. Because of these properties, cast aluminum bronze is used in bearings and pump parts. These alloys are useful in areas exposed to salt water and corrosive gases.
Manganese bronze is an exceptionally high strength, tough, corrosion-resistant copper zinc alloy containing aluminum, manganese, iron, and occasionally, nickel or tin. This metal can be formed, extruded, drawn, or rolled to any desired shape. In rod form, it is generally used for machined parts for aircraft landing gears and brackets.
Silicon bronze is a more recent development composed of about 95 percent copper, 3 percent silicon, and 2 percent manganese, zinc, iron, tin, and aluminum. Although not a bronze in the true sense because of its small tin content, silicon bronze has high strength and great corrosion resistance.
Monel
Monel, the leading high nickel alloy, combines the properties of high strength and excellent corrosion resistance. This metal consists of 68 percent nickel, 29 percent copper, 0.2 percent iron, 1 percent manganese, and 1.8 percent of other elements. It cannot be hardened by heat treatment.
Monel, adaptable to casting and hot or cold working, can be successfully welded. It has working properties like those of steel. When forged and annealed, it has a tensile strength of 80,000 psi. This can be increased by cold working to 125,000 psi, sufficient for classification among the tough alloys.
Monel has been successfully used for gears and chains to operate retractable landing gears and for structural parts subject to corrosion. In aircraft, Monel is used for parts demanding both strength and high resistance to corrosion, such as exhaust manifolds and carburetor needle valves and sleeves.
K-Monel
K-Monel is a nonferrous alloy containing mainly nickel, copper, and aluminum. Adding a small amount of aluminum to the Monel formula produces it. It is corrosion resistant and capable of being hardened by heat treatment.
K-Monel has been successfully used for gears and structural members in aircraft, which are subjected to corrosive attacks. This alloy is nonmagnetic at all temperatures. Both oxyacetylene and electric arc welding have successfully welded K-Monel sheet.
Nickel and Nickel Alloys
There are basically two nickel alloys used in aircraft: Monel and Inconel. Monel contains about 68 percent nickel and 29 percent copper, plus small amounts of iron and manganese.
Nickel alloys can be welded or easily machined. Some of the nickel Monel, especially the nickel Monels containing small amounts of aluminum, are heat-treatable to similar tensile strengths of steel. Nickel Monel is used in gears and parts that require high strength and toughness, such as exhaust systems that require high strength and corrosion resistance at elevated temperatures.
Inconel alloys of nickel produce a high strength, high temperature alloy containing approximately 80 percent nickel, 14 percent chromium, and small amounts of iron and other elements. The nickel Inconel alloys are frequently used in turbine engines because of their ability to maintain their strength and corrosion resistance under extremely high-temperature conditions.
Inconel and stainless steel are similar in appearance and are frequently found in the same areas of the engine. Sometimes it is important to identify the difference between the metal samples. A common test is to apply one drop of cupric chloride and hydrochloric acid solution to the unknown metal and allow it to remain for 2 minutes. At the end of the soak period, a shiny spot indicates the material is nickel Inconel, and a copper-colored spot indicates stainless steel.
