Aluminum Alloys
In the wrought form, commercially-pure aluminum is known as 1100. It has a high degree of resistance to corrosion and is easily formed into intricate shapes. It is relatively low in strength and does not have the properties required for structural aircraft parts. The process of alloying generally obtains high strengths. The resulting alloys are less easily formed and, with some exceptions, have lower resistance to corrosion than 1100 aluminum.Alloying is not the only method of increasing the strength of aluminum. Like other materials, aluminum becomes stronger and harder as it is rolled, formed, or otherwise cold worked. Since the hardness depends on the amount of cold working done, 1100 and some wrought aluminum alloys are available in several strain-hardened tempers. The soft or annealed condition is designated O. If the material is strain hardened, it is said to be in the H condition.
The most widely used alloys in aircraft construction are hardened by heat treatment rather than by cold work. These alloys are designated by a somewhat different set of symbols: T4 and W indicate solution heat treated and quenched but not aged, and T6 indicates an alloy in the heat-treated, hardened condition.
- W—solution heat treated, unstable temper
- T—treated to produce stable tempers other than F, O, or H
- T2—annealed (cast products only)
- T3—solution heat treated and then cold worked
- T4—solution heat treated
- T5—artificially aged only
- T6—solution heat treated and then artificially aged
- T7—solution heat treated and then stabilized
- T8—solution heat treated, cold worked, and then artificially aged
- T9—solution heat treated, artificially aged, and then cold worked
- T10—artificially aged and then cold worked
Additional digits may be added to T1 through T10 to indicate a variation in treatment, which significantly alters the characteristics of the product.
Aluminum-alloy sheets are marked with the specification number on approximately every square foot of material. If for any reason this identification is not on the material, it is possible to separate the heat-treatable alloys from the non-heat-treatable alloys by immersing a sample of the material in a 10 percent solution of caustic soda (sodium hydroxide). The heat-treatable alloys turn black due to the copper content, whereas the others remain bright. In the case of clad material, the surface remains bright, but there is a dark area in the middle when viewed from the edge.
Alclad Aluminum
The terms “Alclad and Pureclad” are used to designate sheets that consist of an aluminum-alloy core coated with a layer of pure aluminum to a depth of approximately 51⁄2 percent on each side. The pure aluminum coating affords a dual protection for the core, preventing contact with any corrosive agents, and protecting the core electrolytically by preventing any attack caused by scratching or from other abrasions.There are two types of heat treatments applicable to aluminum alloys: solution heat treatment and precipitation heat treatment. Some alloys, such as 2017 and 2024, develop their full properties as a result of solution heat treatment followed by about 4 days of aging at room temperature. Other alloys, such as 2014 and 7075, require both heat treatments.
The alloys that require precipitation heat treatment (artificial aging) to develop their full strength also age to a limited extent at room temperature; the rate and amount of strengthening depends upon the alloy. Some reach their maximum natural or room temperature aging strength in a few days, and are designated as –T4 or –T3 temper. Others continue to age appreciably over a long period of time.
Because of this natural aging, the –W designation is specified only when the period of aging is indicated, for example, 7075–W (1⁄2 hour). Thus, there is considerable difference in the mechanical and physical properties of freshly quenched (–W) material and material that is in the –T3 or –T4 temper.
The hardening of an aluminum alloy by heat treatment consists of four distinct steps:
- Heating to a predetermined temperature.
- Soaking at temperature for a specified length of time.
- Rapidly quenching to a relatively low temperature.
- Aging or precipitation hardening either spontaneously at room temperature, or because of a low temperature thermal treatment.
The first three steps above are known as solution heat treatment, although it has become common practice to use the shorter term, “heat treatment.” Room temperature hardening is known as natural aging, while hardening done at moderate temperatures is called artificial aging, or precipitation heat treatment.
Solution Heat Treatment
Temperature
The temperatures used for solution heat treating vary with different alloys and range from 825 °F to 980 °F. As a rule, they must be controlled within a very narrow range (±10 °F) to obtain specified properties.If the temperature is too low, maximum strength is not obtained. When excessive temperatures are used, there is danger of melting the low melting constituents of some alloys with consequent lowering of the physical properties of the alloy. Even if melting does not occur, the use of higher than recommended temperatures promotes discoloration and increases quenching strains.
Time at Temperature
The time at temperature, referred to as soaking time, is measured from the time the coldest metal reaches the minimum limit of the desired temperature range. The soaking time varies, depending upon the alloy and thickness, from 10 minutes for thin sheets to approximately 12 hours for heavy forgings. For the heavy sections, the nominal soaking time is approximately 1 hour for each inch of cross-sectional thickness. [Figure]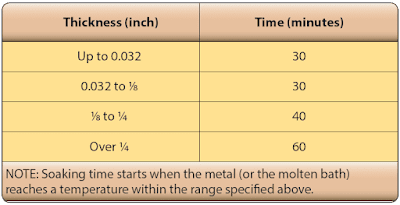 |
| Typical soaking times for heat treatment |
Choose the minimum soaking time necessary to develop the required physical properties. The effect of an abbreviated soaking time is obvious. An excessive soaking period aggravates high-temperature oxidation. With clad material, prolonged heating results in excessive diffusion of copper and other soluble constituents into the protective cladding and may defeat the purpose of cladding.
Quenching
After the soluble constituents are in solid solution, the material is quenched to prevent or retard immediate re-precipitation. Three distinct quenching methods are employed. The one to be used in any instance depends upon the part, the alloy, and the properties desired.Cold Water Quenching
Parts produced from sheet, extrusions, tubing, small forgings, and similar type material are generally quenched in a cold-water bath. The temperature of the water before quenching should not exceed 85 °F.Using a sufficient quantity of water keeps the temperature rise under 20 °F. Such a drastic quench ensures maximum resistance to corrosion. This is particularly important when working with alloys, such as 2017, 2024, and 7075. This is the reason a drastic quench is preferred, even though a slower quench may produce the required mechanical properties.
Hot Water Quenching
Large forgings and heavy sections can be quenched in hot or boiling water. This type of quench minimizes distortion and alleviates cracking, which may be produced by the unequal temperatures obtained during the quench. The use of a hot water quench is permitted with these parts, because the temperature of the quench water does not critically affect the resistance to corrosion of the forging alloys. In addition, the resistance to corrosion of heavy sections is not as critical a factor as for thin sections.Spray Quenching
High-velocity water sprays are useful for parts formed from clad sheet and for large sections of almost all alloys. This type of quench also minimizes distortion and alleviates quench cracking. However, many specifications forbid the use of spray quenching for bare 2017 and 2024 sheet materials because of the effect on their resistance to corrosion.Lag Between Soaking and Quenching
The time interval between the removal of the material from the furnace and quenching is critical for some alloys and should be held to a minimum. The elapsed time must not exceed 10 seconds when solution heat treating 2017 or 2024 sheet material. The allowable time for heavy sections may be slightly greater.Allowing the metal to cool slightly before quenching promotes re-precipitation from the solid solution. The precipitation occurs along grain boundaries and in certain slip planes causing poorer formability. In the case of 2017, 2024, and 7075 alloys, their resistance to intergranular corrosion is adversely affected.
